A Clinical Trial Case Summary: ePRO collection in a multi-center, randomized, phase III trial for thyroid cancer
A clinical debate:
There are now many treatments for cancer. While some can only extend a patient’s life after being diagnosed with advanced disease, treatments for several cancer types aim to cure. When someone is diagnosed with a particular type of tumor there are various local, national or international clinical guidelines that help clinicians and patients decide how to treat it. However, there are sometimes differing interpretations of guidelines and therefore variability in clinical practice. One such example is for treating patients who have well-differentiated thyroid cancer (DTC).
The incidence of DTC is increasing faster than any other tumor type and there are various treatment guidelines⁶. For many years, removing the whole thyroid gland using a procedure called total thyroidectomy (TT) was the standard primary treatment.⁷ᐧ⁸ Patients who undergo TT have an excellent prognosis and the cancer does not come back in the vast majority of patients, so they do not die from the cancer itself. In recent years, several surgeons have begun using hemi-thyroidectomy (HT), partial removal of the thyroid gland, mainly because it is likely to have a lower rate of complications than TT ⁹ᐧ¹⁰. However, there is a concern, particularly among patients, that the chance of the cancer coming back could be higher with HT. There are several published research studies comparing HT with TT, but they have all been observational studies of patient records, often retrospectively done, hence they are not reliable enough to determine which procedure is best¹⁰.
Enter the HoT Trial:
To address the surgical debate, once and for all, over which procedure should be recommended (TT or HT), a group of researchers in the UK have designed and are now conducting the first ever multi-center, randomized, phase III clinical trial in patients with DTC to directly compare HT with TT (HoT trial: Hemi-thyroidectomy Or Total thyroidectomy). The trial will show the pros and cons of each surgical procedure, with the ultimate goals of driving broad consistency in surgical practice for individuals with DTC and allowing patients and their clinician to make a fully informed decision. The results of the trial i) will inform surgical oncologists whether HT is non inferior to TT in low-risk thyroid cancer in terms of cancer recurrence and ii) will compare quality of life and surgical complications between patients who undergo HT versus TT. The trial is led by an expert thyroid surgeon Dr Dae Kim (St George’s Hospital, UK) and Professor Allan Hackshaw (Epidemiologist at University College London, UK) who oversees the trial conduct.
Patient-Friendly Tactics For Patient-Reported Outcomes:
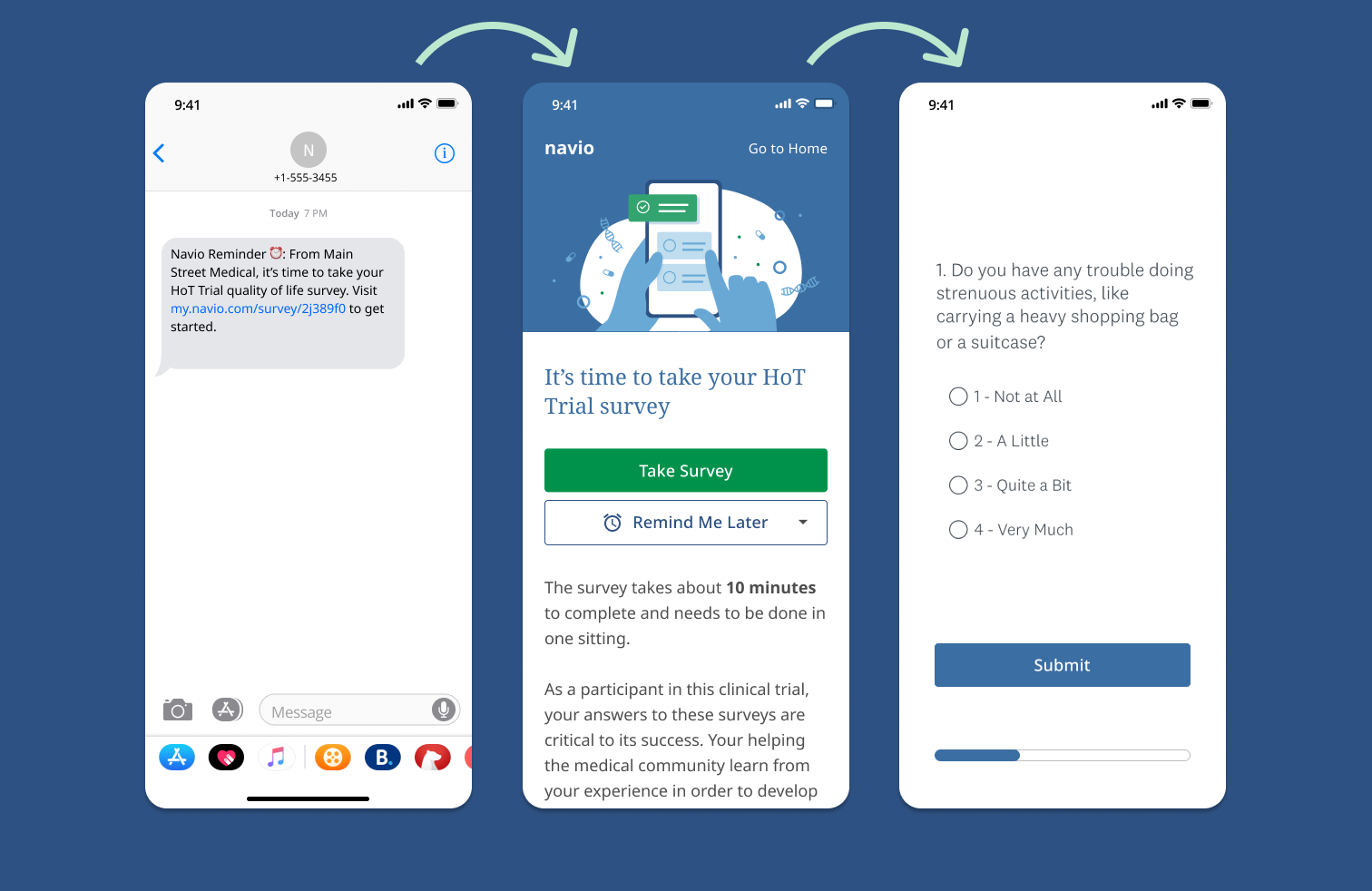
A common challenge in clinical research is the need to gather, analyze, and report complete datasets³. Patient quality-of-life (QoL) is a major endpoint in modern clinical trials, but investigators in many places, including the United Kingdom (U.K.), continue to use paper QoL surveys, which are filled out during a patient’s clinic visit. This takes extra time in the clinic, and patients therefore decline to complete the survey or they do not answer all of the survey questions, both leading to missing data. One attempted solution has been to provide an electronic device, such as a notepad, to the patient so that they can provide data when at home, but this can be an expensive and logistically-involved approach. The majority of individuals in all age groups now own a smartphone, and the idea of using patients’ own smartphones and patient engagement platforms to collect QoL data has more recently been proposed and implemented but is still not commonplace in the U.K. or in the E.U.¹ᐧ² Of note, many of the validated QoL surveys already allow patients to use their own smartphone if approved by the copyright holders of the licensed questionnaires.³ᐧ⁴
Two Birds, One Stone:
Professor Hackshaw and Navio have worked closely together to design and build a nested study into the HoT trial in order to evaluate QoL electronic survey collection methods. It is expected that the elimination of patients having to stay longer in clinic visits and the high accessibility of their own cell phone will result in better adherence to trial survey completion. The HoT Trial nested study will assess the clinical utility of Navio’s application and its patient-reported outcome (PRO) data collection system versus traditional paper surveys, with potential major benefits for patients who take part in clinical trials and study investigators.
Navio’s Quality Solution:
Navio’s smartphone application is a comprehensive oncology patient engagement tool built for investigator-led and pharma-sponsored clinical trials, diagnostic lab products, and healthcare providers' daily workflow. A web-based application with a thoughtful, intuitive, and engaging patient interface, available on both Apple iOS and Android platforms (as well as computer browsers), Navio’s platform utilizes tactics which help patients adhere to clinical trial requirements such as trial medication management, SMS-based text reminders, confirmation for key events, data collection for validated quality of life surveys and customized trial resources. This combination of tactics and design not only streamlines the clinical trial experience for patients, sponsors and investigators, but also supports patients day-to-day by providing a tool for non-trial clinical treatment and management, side-effect reporting, education, and more. Designed for the rigors of clinical use and research in the U.S.A. and abroad, Navio’s platform is GDPR and HIPAA compliant, contains internal auditability for Protected Health Information (PHI) edits and access, and can integrate seamlessly with investigators’, sponsors’, and providers’ own clinical and research platforms. For the complex needs of the HoT trial, the Navio app is fit for the challenge:
“We are one of the largest cancer trials centres in the UK and we aim to incorporate more modern technologies into our studies. The HoT trial is the third major national clinical trial in thyroid cancer, and is an ideal study to evaluate the benefits of patient-centric software. The HoT trial plans to recruit about 500 patients from more than 25 UK cancer centres. We are using established QoL questionnaires specific to cancer patients and those who may develop side effects of thyroid cancer treatments. We will collect QoL data at baseline, after surgery and every few months thereafter for up to 5-6 years. The Navio application has, perhaps, the best interface I have seen, making it easy to use by patients, which is the ultimate goal. In addition, the application allows us to regularly provide information about the trial’s progress directly to patients, thus keeping them engaged throughout the study.”
— Professor Allan Hackshaw, Director of the Cancer Research UK & UCL Cancer Trials Centre
The Future is Bright — and “HoT”:
The HoT Trial, in collaboration with Navio, begins enrolling patients in late fall 2021, with the expectation that trial results will lead to more consensus regarding surgical treatment in DTC and subsequently more consistency in clinical practice. In addition, the nested Navio study will help assess and inform patients’ preferred methods for QoL survey completion, which we believe will lead to a better integration of personal smartphone use and patient engagement platforms into clinical trials.
References:
- Pew Research Center. Mobile Fact Sheet. https://www.pewinternet.org/fact-sheet/mobile/. Accessed April 29, 2019.
- Pugliese L, Woodriff M, Crowley O, Lam V, Sohn J, Bradley S. Feasibility of the Bring Your Own Device Model in Clinical Research: Results from a Randomized Controlled Pilot Study of a Mobile Patient Engagement Tool. Cureus. 2016 Mar; 8(3): e535. doi: 10.7759/cureus.535.
- Byrom B, Doll H, Muehlhausen W, et al. Measurement Equivalence of Patient-Reported Outcome Measure Response Scale Types Collected Using Bring Your Own Device Compared to Paper and a Provisioned Device: Results of a Randomized Equivalence Trial. Value in Health, 2018. https://www.valueinhealthjournal.com/article/ S1098-3015(17)33615-X/pdf.
- Byrom B, Doll H, Muehlhausen W, et al. Measurement Equivalence of Patient-Reported Outcome Measure Response Scale Types Collected Using Bring Your Own Device Compared to Paper and a Provisioned Device: Results of a Randomized Equivalence Trial. Value in Health, 2018. https://www.valueinhealthjournal.com/article/ S1098-3015(17)33615-X/pdf.
- Critical Path Institute. Electronic Patient-Reported Outcome Consortium. https://c-path.org/programs/eproc/ epro-overview/best-practice-documents/. Accessed April 29, 2019
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030:the unexpected burden of thyroid, liver,and pancreas cancers in the United States. Cancer Res. 2014 Jun 1;74(11):2913-2921.
- Cooper DS, et al., Revised American Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer cancer. Thyroid. 2009 Nov;19(11):1167-1214
- Haugen BRM, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE et al., 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2015 Oct 14.
- Cox C, Bosley M, Southerland LB, Ahmadi S, Perkins J, Roman S et al., Lobectomy for treatment of differentiated thyroid cancer: can patients avoid postoperative thyroid hormone supplementation and be compliant with the American Thyroid Association guidelines? Surgery. 2017 Nov 7.
- Luster M, Aktolun C, Amendoeira I, Barczyński M, Bible KC et al., European Perspective on 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: Proceedings of an Interactive International Symposium. Thyroid. 2019 Jan 7. doi: 10.1089/thy.2017.0129.
Written by

Catherine Fine, MS, CGC

Allan Hackshaw, MSc, PHD



